- Context sensitive half-time is deined as the time for the plasma concentration to fall to half of the value at the time of stopping an infusion
- The half time will usually alter in the setting of varying durations of drug infusion
- The higher the ratio of distribution clearance to clearance due to elimination, the greater the range for context-sensitive half-time
- The longest possible context-sensitive half-time is seen when the infusion has reached steady state, when there is no transfer between compartments and input rate is the same as elimination rate
- Draw and label the axes; draw the curve for the drug with the shortest CSHT first before plotting the others
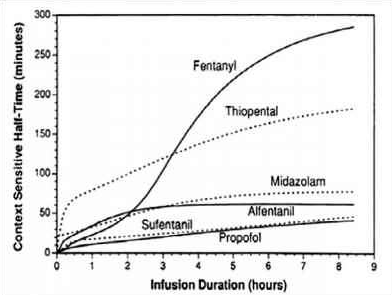
- REMIFENTANIL: Here the elimination always dominates distribution and so there is very little variation in CSHT with time and so it is context insensitive. Draw a straight line starting from the origin and becoming near horizontal after the CSHT reaches 5 min. This demonstrates that the half time is not dependent on the length of infusion as clearance by plasma esterases is so rapid. For remifentanil thelongest possible CSHT is only 8 minutes
- PROPOFOL: For propofol the clearance due to elimination is similar to that for distributioninto the second compartment, so plasma concentration falls rapidly after a propofol infusion mainly due to rapid eliminationwith a smaller contribution from distribution. Propofol is not context insensitive as its CSHT continues to rise; however it remains short even after prolonged infusions. Starting at the origin, draw a smooth curve rising steadily towards a CSHT of around 40 min after 8 h of infusion.
- ALFENTANIL: The curve rises from the origin until reaching a CSHT of 50 minat around 2 h of infusion. Thereafter the curve becomes horizontal. This shows that alfentanil is also context insensitivefor infusion durations of 2 h or longer
- THIOPENTONE SODIUM: The curve begins at the origin but rises more steeply than the others so that the CSHT is 50 min after only 30 min infusion duration. Thecurve should be drawn like a slightly slurred build-up exponential reaching a CSHT of 150 min after 8 h of infusion. As the CSHT continues to rise, thiopental does not become context insensitive
- FENTANYL: The most complex curve begins at the origin and is sigmoid in shape. It should cross the alfentanil line at 2 h duration and rise to a CSHT of 250 min after 6 h of infusion. Again, as the CSHT continues to rise, fentanyl does not become context insensitive.
- The maximum possible CSHT for propofol is about 20 minutes, compared with 300 minutes for fentanyl
- It is important to realize that the CSHT does not predict the time to patient awakening but simply the time until the plasma concentration of a drug has fallen by half. The patient may need the plasma concentration to fall by 75% in order to awaken, and the time taken for this or any other percentage fall to occur is known as a decrement time.
- Decrement time: The time taken for the plasma concentration of a drug to fall to the specified percentage of its former value after the cessation of an infusion designed to maintain a steady plasma concentration (time). The CSHT is, therefore, a form of decrement time when the ‘specified percentage’ is 50%.
- Although the CSHT for propofol has a maximum value of about 20 minutes, during long, stimulating surgery infusion rates will have been high and the plasma concentration when wake-up is required may be very much less than half the plasma concentration at the end of the infusion. Thus time to awakening using propofol alone may be much longer than the CSHT. This is why the TCI pumps display a decrement time rather than a CSHT.
- When using propofol infusions, the decrement time is commonly quoted as the time taken to reach a plasma level of 1.2 μg.ml−1, as this is the level at which wake up is thought likely to occur in the absence of any other sedative agents.
- It must be remembered that after one CSHT, the next period of time required for plasma concentration to halve again is likely to be much longer. This relects the increasing importance of the slower redistribution and metabolism phases that predominate after re-distribution has taken place. This explains the emphasis on half-time rather than halflife: half-lives are constant whereas half-times are not!